Deflux is made of frequently used biocompatible materials with an established safety profile
Deflux is a biocompatible, biodegradable, nonmigratory viscous gel that shows no signs of mutagenesis and has an established safety profile. Deflux is indicated for vesicoureteral reflux (VUR) grades II-IV in children. Deflux is injected by inserting a cystoscope through the urethra into the bladder and depositing the biocompatible gel into the mucosa of the ureteral tunnel creating ureteral tunnel and orifice coaptation.
The natural solution
Deflux consists of two biocompatible polysaccharides: Non-Animal Stabilized Hyaluronic Acid (NASHA®) and Dextranomer (Dx).1
Non-Animal Stabilized Hyaluronic Acid (NASHA)
- Has been used for over two decades in more than 50 million procedures worldwide2
- Undergoes a patented process to form a gel with increased viscosity and stability3
- The hyaluronic acid is synthesized by cultured non-animal sourced bacteria resulting in a pure and consistent preparation free from contaminants and viruses3
- High molecular weight increases durability within the body allowing for long-term correction of VUR3
Dextranomer (Dx) Microspheres
- Cross-linked polymer of dextran3
- Microspheres measure between 80 μm and 250 μm3
- Large size prevents any risk of migration from site of injection3
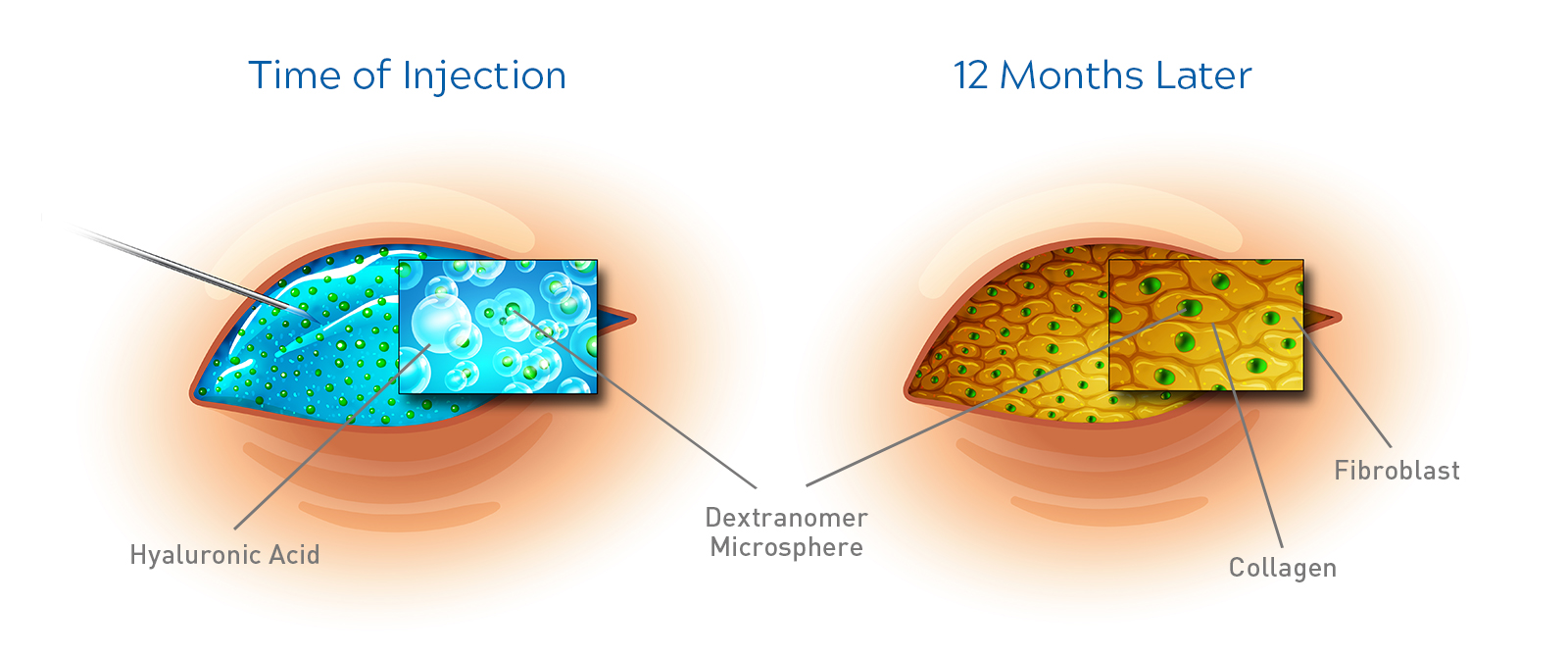
The Deflux implant is stable, remains in position, and does not disappear over time4
Following the injection of Deflux gel, fibroblast cells have been shown to infiltrate the implant and migrate between dextranomer microspheres. A matrix of collagen is then generated, which surrounds the microsphere. This collagen matrix effectively replaces the NASHA component of the implant.